|
|
Université
catholique de Louvain
Louvain Drug Research Institute > Cellular
and Molecular Pharmacology |
|
|
Clinical
evaluation of new therapeutic approaches |
Team
- Senior investigators:
P.M. Tulkens, F.
Van Bambeke
- Post-doctoral fellows:
I. Delattre (2013-), C. Mark (2016-)
- Doctoral fellows:
A. Miranda Bastos (2010-2016), P. Ngougni Pokem (2015-)
- Former investigators:
S.
Ibrahim (1984-1990), P. Lambricht (1986-1991), C. Bruno (1994-1996), E. Viaene
(2000-2002), H. Servais (2002), V. Basma (2003-2004), S. Carryn (2003-2009),
E. Ampe (2005-2013), K. Berthoin (2006-2009), S. Carbonnelle (2008-2010)
Collaborations
- Cliniques
universitaires St-Luc, UCL, Bruxelles
- Cliniques
universitaires de l'UCL à Mont-Godinne, Yvoir
- Hôpital
Erasme, ULB, Bruxelles
- Hôpital
St. Pierre,
Bruxelles
- Universitair
Ziekenhuis, VUB, Bruxelles (Jette)
- Centre
hospitalier universitaire,
ULg, Liège
- Universitair
Ziekenhuis, Ugent, Gent
- Akademische
Ziekenhuis, Brugge
- Onze
Lieve Vrouw Ziekenhuis, Aalst
Main
research programs
The final and the most important aspect
in drugs development is their clinical use. Pharmacology and toxicology have
therefore an obvious application which is the definition of the proper use of
drugs, and their permanent improvement. In this context, we use the knowledge
gained by our studies on the pharmacology of antiinfective drugs to design and
perform clinical trials directed to these goals. These studies attempt to apply
to the clinical environment the concepts of pharmacodynamics and toxicology
which were proven useful in our experimental studies. We also set up the necessary
monitoring procedures when these require non-routine investigations or developments.
In this context, the following clinical evaluations have been or are being
performed:
Administration of aminoglycosides in a "once-a-day" schedule
and evaluation of renal alterations
This
new model of administration was proposed on the basis of experimental studies
demonstrating its potential for being more, or at least as effective than the
conventional schedules (3 times a day), while causing less toxic reactions.
The lower toxicity stems from the saturable character of aminoglycoside uptake
by target tissues (kidney, inner ear). Moreover, the toxicity induced by aminoglycosides
proceeds by successive thresholds from recoverable alterations towards irreversible
damage. Keeping the level of alterations below a critical threshold results
therefore in an effective protection of the patient.
Our laboratory was a pioneer in the demonstration of administering aminoglycoside
once-a-day. In particular, we
developed an assay applicable
in the clinic for the early and non
invasive detection of aminoglycoside nephrotoxicity.
It consists in measuring urinary phospholipidsas an hallmark of early leasions
induced in the kidney cortex (phospholipidosis; see further details in the section
on cellular toxicity).
Results show that phospholipiduria is a useful marker of impending toxicity
in correlation with the duration and extent of treatment (Figure 1). It is also
a useful monitoring criteria for assessing the safety of new schedules. It can
also be used experimentally to assess the effectiveness of nephroprotectants.
This
once-a-day schedule of administration was successfully tested in several patient
populations and is now introduced in a large number of applications of these
drugs.
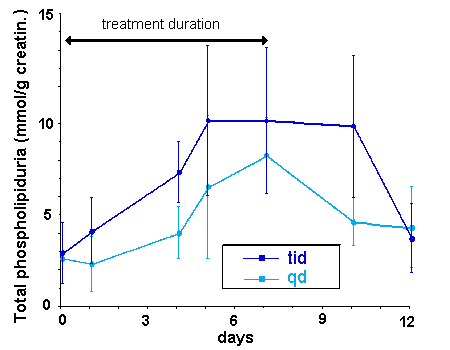 |
Figure
1
Phospholipiduria as an early indicator of aminoglycoside-induced
nephrotoxicity. The figure compares the level of urinary phospholipids
in cancer patients receiving 6 mg/kg netilmicin daily as a
single injection (qd)
or divided in 3 injections over each 24 h period (tid)
during 7 days.
Phospholipiduria
rises steadily during the treatment period, but returns to normal
values within 5 days of treatment discontinuation.
Despite the fact that the interindividual variations are important,
it clearly appears that the tid administration causes a more
rapid and elevated phospholipidurva than the qd
administration. The rise caused by tid treatment shows also a slower reversibility
From
Van der Auwera et al, 1991
|
|
In
parallel, experimental and clinical studies have been initiated to develop the
use of apoptosis markers for the study of aminoglycoside-induced toxicity.
Selected References
(by reverse chronological order;
for a full reference list, see our publication list)
- S. Ibrahim, J.P. Langhendries,
A. Bernard and P.M. Tulkens. 1994. Urinary phospholipids excretion in neonates
treated with amikacin. Int. J. Clin. Pharm. Res. 14:149-156.
- J.P. Langhendries, O.
Battisti, J.M. Bertrand, A. François, J. Darimont, S. Ibrahim and E.
Scalais. 1993. Once-a-day administration of amikacin in neonates: assessment
of nephrotoxicity and ototoxicity. Dev. Pharamacol. Ther., 120:220-230
- P.
Van der Auwera, F. Meunier, S. Ibrahim, L. Kaufman, M.P. Derde, P.M. Tulkens.
1991. Pharmacodynamic parameters and toxicity of netilmicin (6 mg/kg.day)
given once daily or in three divided doses to cancer patients with urinary
tract infection. Antimicrob Agents Chemother 35:640-647.
- P.M. Tulkens. 1991. Pharmacokinetic
and toxicological evaluation of the once-a-day regimen versus conventional
schedules of netilmicin and amikacin. J. Antimicrob. Chemother. 27(c):49-61.
- S. Ibrahim, B.K. Kishore,
P. Lambricht, G. Laurent and P.M. Tulkens. 1991. Effect of aminoglycosides
and of coadministration of poly-l-aspartic acid on urinary phsopholipids excretion:
a comparative study. In: P.H. Bach, N.J. Gregg,M.F. Wilks and l. Delacruzz,
(eds). Nephrotoxicity:mechanisms, early diagnosis and therapeutic management.
Marcel Dekker, New York, 105-109.
- S. Ibrahim, M.P. Derde,
l. Kaufman, f. Clerckx-Braun , Ph. Jacqmin, J. Donnez and P.M. Tulkens. 1990.
Analysis of safety, pharmacokinetics and efficacy of once-a-day administration
of netilmicin and amikacin vs their conventional schedules in pelvic inflammatory
disease patients. Renal failure 12:199-203.
Evaluation
of beta-lactam
antibiotics in special applications in relation with their pharmacodynamic properties
In contrast with aminoglycosides,
beta-lactam antibiotics must be administered several times a day, because their
serum concentration must ideally remain above the MIC of the offending organisms
for the whole period of treatment. While this can be easily achieved in simple
applications for these drugs, little is known concerning this aspect in special
situations where pharmacokinetic parameters can vary largely from what is observed
in 'normal' patients. In this context, we have examined the stability of beta-lactams
in their conditions of use over time in order to define in which conditions
they can be used for continuous infusion (stability over time and at increasing
temperatures [To comply with the European Pharmacopoeia, beta-lactam solutions
should always contain at least 90% of intact molecule]; compatibility with other
drugs in solutions of perfusion).
| beta-lactam |
concentration |
time during
which the drug remains stable (> 90 % recovery) at 25°C |
| imipenem |
0.8
%
|
3.5
h
|
| meropenem |
1
%
|
24
h
|
|
4
%
|
12
h
|
|
6.4
%
|
<
6 h
|
| doripenem |
1
%
|
24
h
|
|
4
%
|
>
18 h
|
|
6.4
%
|
<
6 h
|
| piperacillin |
13
%
|
>
24 h
|
| temocillin |
8
%
|
24
h
|
| ceftazidime |
12
%
|
24
h
|
| cefepime |
12
%
|
>
24 h
|
| aztrenam |
10
%
|
>
24 h
|
|
We are now evaluating the
pharmacokinetics and pharmacodynamics of beta-lactams in specific populations
(intensive care, hemodialysis, pediatrics, e.g). in order to propose optimal
dosages. In the frame of a FP7 European
project, we are also developing a point-of-care approach consisting of an
integrated diagnostic tool allowing to measure in real time the free fraction
of beta-lactams and proposing dose adjustments based on pharmacodynamic algorithms
in order to optimize the chance of therapeutic success while at the same time
reducing the risk of selecting resistance
Selected References (by
reverse chronological order; for a full reference list, see our publication
list)
- Laterre PF, Wittebole
X, Van de Velde S, Muller AE, Mouton JW, Carryn S, Tulkens PM, Dugernier T.
Temocillin 6 g daily in critically-ill patients: continuous infusion versus
thrice daily administration
Journal of Antimicrobial Chemotherapy (2015) 70:891–89. (PDF)
- Frippiat F, Musuamba
PT, Seidel L, Albert A, Denooz R, Charlier C, Van Bambeke F, Wallemacq P,
Descy J, Lambermont B, Layios N, Damas P, Moutschen M. Modelled target attainment
after meropenem infusion in patients with severe nosocomial pneumonia: the
PROMESSE study. Journal of Antimicrobial Chemotherapy (2015) 70:207-216. (PDF)
- Vandecasteele SJ, Miranda
Bastos AC, Capron A, Spinewine A, Tulkens PM, Van Bambeke F. Thrice weekly
temocillin administered after each dialysis session is appropriate for the
treatment of serious Gram-negative infections in haemodialysis patients. International
Journal of Antimicrobial Agents (2015) 46:660-665. (PDF)
- Berthoin
K, Le Duff CS, Marchand-Brynaert J, Carryn S, Tulkens PM. 2010. Stability
of meropenem and doripenem solutions for administration by continuous infusion
Journal of Antimicrobial Chemotherapy 65:1073-1075. (PDF)
- Carryn S, Couwenbergh B, Tulkens
PM. 2010. Long-term Stability
of Temocillin in Elastomeric Pumps for Outpatient Antibiotic Therapy (OPAT)
in Cystic Fibrosis (CF) Patients.
Journal
of Antimicrobial Chemotherapy 65:2045-2046.(PDF)
- De Jongh R, Hens R, Basma
V, Mouton JW, Tulkens PM, Carryn S. 2008. Continuous vs intermittent infusion
of temocillin, a directed spectrum penicillin for intensive care patients
with nosocomial pneumonia: stability, compatibility, population pharmacokinetic
studies and breakpoint selection. Journal of Antimicrobial Chemotherapy 61:382-388.
(PDF)
- Baririan N, Chanteux
H, Viaene E, Servais H, Tulkens PM. 2003. Stability and compatibility of cefepime
in comparison with ceftazidime for administration by continuous infusion for
ambulatory treatment of cystic fibrosis patients and for administration in
intensive care units (abridged title). Journal of Antimicrobial Chemotherapy
51:651-658. (PDF)
- Viaene E, Chanteux H,
Servais H, Mingeot-Leclercq MP, Tulkens PM. 2002. Comparative stability studies
of antipseudomonal beta-lactams for potential administration through portable
elastomeric pumps (home therapy for cystic fibrosis patients) and motor-operated
syringes (intensive care units). Antimicrobial Agents and Chemotherapy 46:2327-2332.
(PDF)
- Servais H, Tulkens PM.
2001. Stability and compatibility of ceftazidime administered by continuous
infusion to intensive care patients. Antimicrobial Agents and Chemotherapy
45:2643-2647 (PDF)
Evaluation
of vancomycin administration by continuous infusion
For glycopeptides
like vancomycin, efficacy is best predicted by the ratio between the Area-Under-the-concentration-Curve
(AUC) and the MIC of the offending organism. Optimisation of AUC can be easily
achieved using continuous infusion. This mode of administration facilitates
therapeutic monitoring and allows for rapid readjustement of dosage to achieve
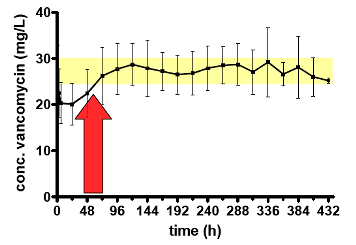
the target concentration. We have evaluated the feasibility of continuous infusion
of vancomycin in the clinics by following the serum levels (both total and free
drug). We have examined whether the dose selected allowed to reach the pharmacodynamic
target (AUC/MIC > 400) taking into account the MIC of the bacteria isolated
in these patients. We found a high variability in serum levels and in vancomycin
free fractions among patients, suggesting the importance of monitoring serum
levels and adapting doses in fonction of the MIC of the isolated pathogen.
 |
 |
|
Figure 2
Left: mean
serum level of vancomycin in 54 patients treated by continuous infusion,
with daily readjustement of doses based on therapeutic monitoring.
The yellow zone highlights the target concentration. The red arrow
points to the fact that 48h are needed to adjust the infusion rate
so as to reach the target level.
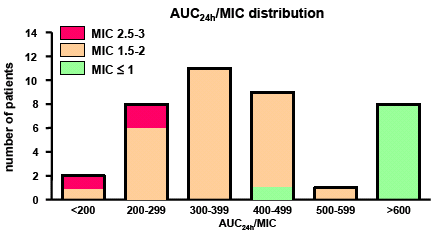
Right:
pharmacodynamic assessment. Patients are categorized according to
the AUC/MIC ratio calculated based on pharmacokinetic and microbiological
data. The graph shows that reaching the pharmacodynamic target (AUC/MIC
> 400) is unlikely in patients infected by bacteria with MIC
> 2 mg/L
From Ampe
et al., 2013
|
|
Selected References (by
reverse chronological order; for a full reference list, see our publication
list)
- Ampe
E, Delaere B, Hecq JD, Tulkens PM, Glupczynski Y. Implementation of a protocol
for administration of vancomycin by continuous infusion: pharmacokinetic,
pharmacodynamic, and toxicological aspects. International Journal of Antimicrobial
Agents (2013) 41:439-446. (PDF)
- E. Ampe, K. Berthoin,
B. Delaere, S. Noirhomme, P. M. Tulkens, J. Hecq, S. Carryn, Y. Glupczynski.
2008. Assay of free and total vancomycin serum concentrations with E-test-based
determination of MICs in patients treated by continuous infusion for severe
staphylococcal and enterococcal infections. 48th Interscience Conference on
Antimicrobial Agents and Chemotherapy (ICAAC) & 46th Annual meeting of
the Infectious Diseases Society of America (IDSA). Washington, DC, Poster
no. A-1882 (PDF)
Expertises
- Clinical trials of new drugs and new schemes of administrations.
- Assay of antibiotics
in biological fluids
- Studies of antibiotic
stability and compatibility in their conditions of use in the clinics
- Monitoring of renal alterations by non-invasive approaches.
Additional
information: <tulkens@facm.ucl.ac.be>
Last significant update: May 2016